UH Seidman Cancer Center Offers Patients Rare Access to New Kidney Cancer Tracer
June 27, 2024
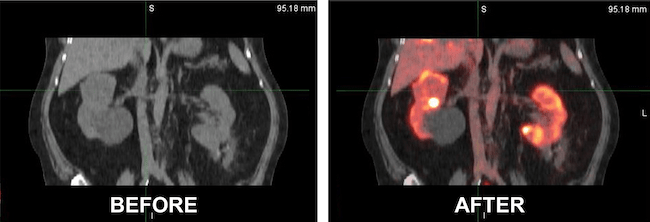
CLEVELAND – University Hospitals (UH) Seidman Cancer Center is one of just a few cancer centers nationwide to offer its patients early access to a novel and more sensitive method of diagnosing kidney cancer. UH care teams are giving patients a special contrast specific to kidney cancer in conjunction with a PET scan, in advance of expected approval by the FDA at the end of the year. Adult patients with renal masses >7 cm are eligible to participate.
“Compared to the normal scans, which can include CT scans or MRI or ultrasound, this PET scan with this contrast or tracer has a much higher sensitivity and specificity to find kidney cancer, particularly clear cell renal cell carcinoma in the kidney. The new contrast, given a few days before the actual PET scan, is an effort to improve diagnostic options for kidney cancer,” says Pedro Barata, MD, MSc, FACP, Director of the Clinical Genitourinary Medical Oncology Research Program and Miggo Family Chair in Cancer Research. He’s also leading the implementation of the novel tracer at UH Seidman Cancer Center.
“PET scans are available for many tumors, but for some tumors like kidney cancer, the tracer we normally use is FDG, which tests glucose metabolism,” he says. “Its role in kidney cancer is limited. We needed a kidney cancer-specific tracer."
Researchers hit upon radiolabeled girentuximab (TLX250-CDx), a monoclonal antibody that targets carbonic anhydrase IX (CA9), a protein expressed in clear cell renal cell carcinoma. Results from the ZIRCON Phase 3 clinical trial, which enrolled 300 patients from 36 sites in nine countries, show that the new tracer and PET scan are an improvement over current scans used for patients with renal tumors. Sensitivity and specificity averages across all three readers were 86% and 87%, respectively, confirming that the new process improves identification of clear cell renal carcinoma compared to conventional cross-sectional approaches. In addition, the clinical trial confirmed the safety and tolerability profile of TLX250-CDx.
Dr. Barata says. “It finds the molecule CA9, expressed by kidney cancer in the entire body, allowing us to have a more complete and nuanced view of the extent of disease. This tracer is the first and likely best kidney cancer-specific tracer we know of thus far. We've never been this close to a scan that can detect with the highest sensitivity not only kidney cancer in the kidney, but also outside the kidney.”
“Any cancer has the ability to leave the kidney and go to other places in the body,” he adds. “Having a scan and a tracer that improves detection of this cancer it’s an advantage. We can adapt our treatment to those patients in a much more accurate manner. I’m pleased to say we have this new option available here at University Hospitals Seidman Cancer Center.
Tags: Cancer, Innovation, Technology


